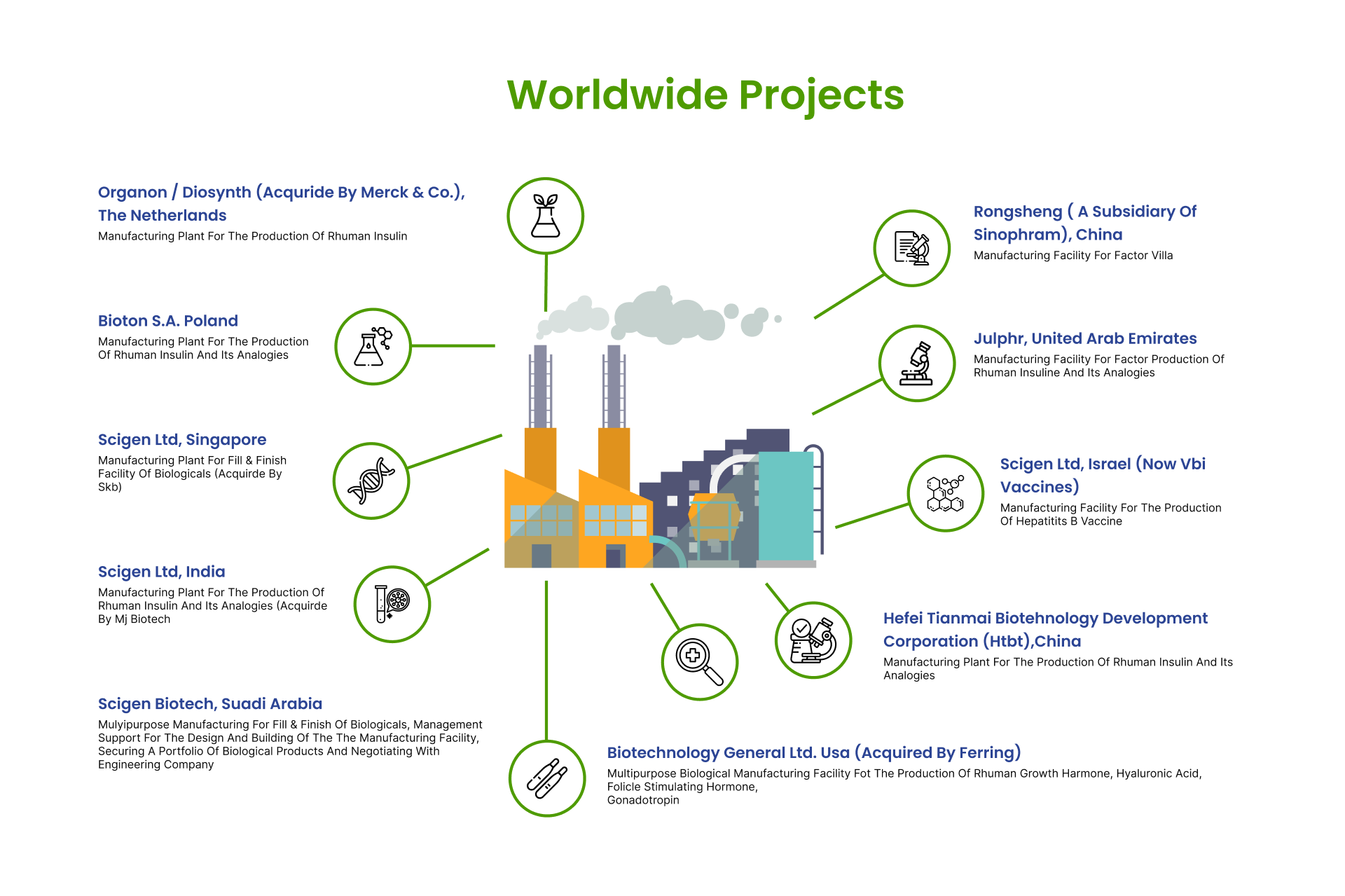
Development And Technology Transfer Of Innovative Biotechnology Products
Development and building state-of-the-art biologic manufacturing facilities. Joint development and commercialization with worldwide partners. Together with our engineering partner, we provide global design and construction for biologicals, pharmaceuticals and API, from concept to turnkey manufacturing facilities and systems approvable by highly rated health regulatory agencies such as EMA and USFDA.

Development And Technology Transfer Of Innovative Biotechnology Products
Development and building state-of-the-art biologic manufacturing facilities. Joint development and commercialization with worldwide partners. Together with our engineering partner, we provide global design and construction for biologicals, pharmaceuticals and API, from concept to turnkey manufacturing facilities and systems approvable by highly rated health regulatory agencies such as EMA and USFDA.

Development And Technology Transfer Of Innovative Biotechnology Products
Development and building state-of-the-art biologic manufacturing facilities. Joint development and commercialization with worldwide partners. Together with our engineering partner, we provide global design and construction for biologicals, pharmaceuticals and API, from concept to turnkey manufacturing facilities and systems approvable by highly rated health regulatory agencies such as EMA and USFDA.